Cancer Screening in Older Persons
This is the fourth article in a continuing series of articles on cancer in older adults. The goal of this series is to highlight how diagnosis and management of cancer in older adults is different from that in younger patients. The previous article in the series, “Palliative Care in Advanced Cancer in Older Adults: Management of Pain, Fatigue, and Gastrointestinal Symptoms,” was published in the November 2011 issue of Clinical Geriatrics®.
Cancer is a leading cause of morbidity and mortality in the United States,1 especially among adults 65 years and older, as nearly 60% of all cancer diagnoses and 70% of cancer deaths occur in this population.2 The geriatric age demographic is expanding dramatically, and by 2030, one in five Americans will be 65 years or older.3 Longevity has also been increasing, along with the prevalence of illnesses like cancer that are more common with age. As longevity and the proportion of elderly Americans continue to grow, the magnitude of the cancer problem is also expected to grow, with older adults bearing most of the additional burden.
In an attempt to approach these issues proactively, more attention is being paid to screening older adults for cancer.2 Although substantial evidence supports screening adults in their fifth and sixth decades of life for colorectal, breast, and cervical cancers, relatively few screening trials have included participants over 70 years of age.4-7 Therefore, in developing current screening guidelines—the majority of which make age-based recommendations—panels extrapolated these data to older adults. The population of elderly adults is heterogeneous, however, complicating such extrapolations.
Elderly individuals differ with respect to life expectancy, number and severity of comorbidities, functional status impairments, and treatment preferences. As such, an individualized approach to cancer screening is appropriate. We summarize existing guidelines for screening the elderly population for breast, colon, prostate, and cervical cancers (Table 1) and outline geriatric issues to consider when making screening recommendations for an older patient.4-16

General Cancer Screening Considerations
The rationale behind cancer screening, which uses diagnostic studies to identify cancer in asymptomatic individuals, is that detecting cancer at an earlier stage will lead to better outcomes. Detecting disease in its preclinical phase theoretically allows enough time to institute therapies more likely to be effective than treatments undertaken after symptoms arise. For most cancers, treatment options and prognosis depend on the stage of the disease at diagnosis.
Currently, evidence supports using mammography to screen for breast cancer; fecal occult blood testing (FOBT), colonoscopy, or flexible sigmoidoscopy to screen for colon cancer; and Papanicolaou (Pap) smears to screen for cervical cancer. Not enough clear evidence is available to recommend or refute routine prostate cancer screening using prostate- specific antigen (PSA) testing or digital rectal examination (DRE) alone or together in the general male population.8
The screening tool and disease type are important factors in determining the appropriateness of screening (Table 2). A screening tool for cancer should be sensitive in its ability to detect disease in its preclinical phase and have a low false-positive rate. The test should be accessible and acceptable to patients, as well as relatively inexpensive and safe. A disease worth screening for should be highly prevalent and impose a significant personal or monetary burden on society, via high rates of morbidity or mortality or great financial costs. Diseases considered good candidates for screening have a lengthy preclinical phase, have effective treatment options available, and have a natural history that enables enough time for therapeutic interventions to be successfully implemented.

Certain individual attributes increase cancer risk and can help determine whether someone is an appropriate candidate for cancer screening. Factors that place someone at higher risk of cancer and indicate high priority for screening include a personal history of cancer or precancerous lesions, a family member with a history of cancer, or a genetic predisposition to cancer. In 2011, a study by Ziogas and colleagues17 found that a patient’s family history of cancer is rarely updated subsequent to an initial physician’s visit, and the authors recommended doing so every 5 to 10 years to ensure the patient receives appropriate cancer screening advice.
Continued on next page
Screening Older Adults for Cancer
The heterogeneity of the aging process complicates efforts to determine the appropriateness of cancer screening in older adults, as health status varies tremendously among people of the same age. Certain age-related criteria support screening an elderly patient for cancer, whereas others contraindicate screening. In general, characteristics that support screening include a life expectancy of more than 5 to 10 years, few or moderate comorbidities, good functional status, and a strong feeling by that individual that he or she will benefit either through peace of mind or better quality of life. There are at least five specific questions to consider when determining the appropriateness of screening an older patient for cancer9:
• Is the patient likely to die with or of the cancer for which screening is being considered?
• Is the patient likely to live long enough to be affected by the cancer for which screening is being considered?
• Can the patient tolerate treatment of the cancer for which screening is being considered?
• How would treating the cancer affect the patient’s quality of life given his or her coexisting illnesses and geriatric issues?
• Is the patient likely to be harmed by the screening procedure?
Life Expectancy
Persons with limited life expectancy are unlikely to benefit from screening and are more likely to be harmed by complications from screening, diagnostic, and therapeutic procedures or to receive treatment for a clinically unimportant disease.2,10 Therefore, it is important to consider whether a positive screening result is likely to lead to a clinically significant outcome during the patient’s lifetime.
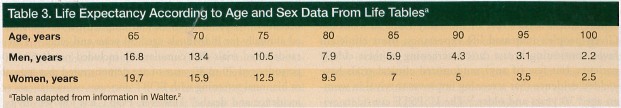
To guide physicians in answering this question, Walter and Covinsky2 published a framework that involves considering the patient’s estimated life expectancy, functional impairments, number and severity of comorbid conditions, and lifetime risk of dying of the cancer. In developing the framework, the authors considered evidence from randomized trials that show that patients with less than 5 years to live are unlikely to recognize a survival benefit from cancer screening. The authors note that the general life expectancy for many elderly men and women exceeds 5 years (Table 3).2 For example, in the general population, men aged 65 years have a life expectancy of approximately 17 years, compared with 20 years for women of that age; a 75-year-old man may expect to live on average 11 additional years, compared with 13 for 75-year-old women; and an 85-year-old man has an average life expectancy of 6 years, compared with 7 for women. In older adults, however, individual health status has a significant effect on life expectancy. For instance, an 85-year-old man in the upper quartile healthwise may have a life expectancy of nearly 9 years, which is longer than a 70-year-old man who has multiple comorbidities. The substantial variability in life expectancy among older persons of the same age exemplifies why physicians should not base decisions on age alone.
Effects of Comorbidity
The prevalence of various chronic conditions, or comorbidities, increases with age.11 As individuals age, they also tend to have a greater number of comorbid conditions that are increasingly severe. Because comorbid conditions negatively affect life expectancy, they play a role in assessing an individual’s risk of dying of cancer. For instance, women with two or more comorbidities in addition to breast cancer have a 20-fold greater chance of dying from causes other than their breast cancer.2 A 75-year-old woman with breast cancer who has relatively few medical problems is more likely to die of breast cancer than a 65-year-old women with breast cancer who also has diabetes, chronic renal insufficiency, and coronary artery disease. Because the younger woman has a higher likelihood of dying of causes other than her breast cancer, she is arguably less likely to benefit from breast cancer screening.
Although comorbidities and cancer are often competing causes of mortality, comorbidities can also increase the risk of cancer mortality. In patients with the same type of cancer who receive the same treatment, those with a greater level of comorbid impairment have worse survival rates than those with no comorbid conditions.12-14 Not only does comorbidity compromise the effectiveness of treatment, it can alter the selection or course of cancer treatment, contributing to worse outcomes. For patients with conditions like severe congestive heart failure, diabetes with end-organ damage, end-stage renal disease, severe chronic obstructive pulmonary disorder, or severe dementia, the level of comorbid impairment may obviate any possible benefit from cancer screening.
No gold standard or universally accepted assessment tool is available to measure the effects of comorbidities on patients with cancer. However, previous and ongoing research has validated a number of tools for use in these patients, including the Charlson Comorbidity Index, the Cumulative Illness Rating Scale,15 and the Kaplan Feinstein Index.
Functional Status
Although it is common for older adults to have multiple comorbidities, functional limitations may more accurately predict life expectancy, especially in patients older than 80 years.18 In older adults with cancer, functional status is considered an independent predictor for mortality and should be evaluated separately from comorbidity.19
Functional status is commonly assessed using two geriatric scales: Activities of Daily Living (ADLs) and Instrumental Activities of Daily Living (IADLs). The percentage of elderly persons requiring assistance increases with age, with over 9% of persons aged 60 to 75 years requiring some form of assistance, compared with nearly 50% of those 85 years and older.20 Dependence in one or more ADLs or IADLs (with the exceptions of toileting, laundry, and housecleaning) may be associated with a 2-year mortality rate ranging from 16% to 40%,21 suggesting that patients who have multiple areas of dependence are less likely to benefit from cancer screening.
Risks and Benefits of Screening
Clinicians should not overlook the several important, potential deleterious effects of cancer screening. All screening tests have a risk of false-positive results, overdiagnosis, additional expense, and increased anxiety and discomfort for patients. The risk of such potential harms is likely even more significant in elderly adults. When screening with mammography, false-positives are common. A historical meta-analysis reported that the probability of false-positive screening mammography ranges from 1% to 6%.22 When taken every year for 10 years, the cumulative risk can be as high as 49%. False-positive screening tests lead to unnecessary secondary tests, biopsies, potentially unnecessary surgeries, added expense, as well as stress and anxiety. For example, in a review of community-dwelling, nursing-home–eligible older women undergoing state-mandated mammography, researchers found that 17% experienced burden and 42% reported pain, anxiety, or depression related to screening that was severe enough to document in the medical record.23
Many forms of cancer screening are associated with the risk of overdiagnosis. Overdiagnosis is the diagnosis of a disease that will never cause symptoms or death during a patient’s lifetime, but results in unnecessary testing and treatment and psychological harm. This effect is likely to be more significant in older individuals due to their decreased life expectancy. For example, colonoscopy for colorectal cancer screening is able to detect a precursor disease decades before it becomes clinically significant. More than 95% of colorectal cancers start as benign adenomatous polyps, but malignant transformation usually takes 10 years or longer.24 As a result, older adults who undergo routine screening for polyps may undergo invasive intervention for a condition they would never have developed and that is not likely to cause their death.
Advanced age is associated with an increased risk of adverse events during screening. For example, a recent meta-analysisshowed that older patients have an increased risk of perforation during colonoscopy, compared with younger patients.25 Specifically, individuals older than 80 years were 60% more likely to experience perforation than patients younger than 80 years. This highlights the importance of considering the risks of screening procedures when assessing the need for cancer screening in older adults.
Continued on next page
Breast Cancer
Other than being a woman, age is the most important risk factor for breast cancer. Over the past decade, mortality rates from breast cancer have declined substantially.9 This decline in mortality is largely attributed to advances in adjuvant treatment, but detecting cancers earlier with routine screening has also likely contributed.26 Screening recommendations for breast cancer largely emphasize mammography because it is the most validated screening tool.27 There is a general consensus that breast cancer screening improves survival for women aged 50 to 70 years. Many clinical trials demonstrate this survival benefit to range between 20% to 40%.28-30 The US Preventive Services Task Force (USPSTF) recommends women aged 50 to 74 years undergo mammography every 2 years. Because no randomized trial to date has provided data supporting breast self-examination (BSE), the USPSTF recommends against teaching it to women.31 The American Cancer Society (ACS) recommends women start mammography screening and clinical breast examinations at age 40 and that they undergo screening annually,7 rather than every 2 years as recommended by the USPSTF, for as long as the woman is in good health. Unlike the USPSTF, the ACS does not dismiss BSE and recommends discussing the risks and benefits of BSE with patients who express interest in performing the procedure.7
Elderly women have a higher incidence of breast cancer and a higher rate of mortality from this disease. Given people’s increased life expectancy, most clinicians favor continuing breast cancer screening in their elderly patients, but few (if any) randomized clinical trials have yielded evidence that screening improves survival in women aged 70 years and older.32 The USPSTF considers evidence for or against mammography for women aged 75 years and older to be inconclusive.31 ACS guidelines do not suggest an age at which to discontinue mammography screening, but they do recommend limiting it to women in good health who are candidates for breast cancer treatment.7 The American Geriatrics Society (AGS) encourages screening for women younger than 85 years of age who have a life expectancy of more than 5 years and for women over the age of 85 who have excellent functional status and feel strongly about its benefits.16
In older women, breast cancer is more likely to be hormone-receptor positive, less aggressive, and better differentiated, and it tends to proliferate far more slowly.33 Mammography and clinical breast examinations are more sensitive tools as a woman ages due to a decline in breast tissue density and increase in fat. Decision aids that explain the pros and cons of continued mammography screening have been shown to be feasible interventions to aid older women in making choices about ongoing screening that are consistent with their values and preferences.34 Due to changes in breast cancer and the effectiveness of screening tools associated with advancing age, it is generally agreed that breast cancer screening should be limited to women with a life expectancy of 5 to 10 years.2,35,36
Colorectal Cancer
The prevalence of colorectal cancer increases considerably as people age, and it is a major source of cancer mortality in elderly persons.37 Colorectal cancer screening decreases the risk of mortality from colorectal cancer by allowing lesions to be detected at an earlier, more treatable stage and, in the case of colonoscopy, allowing identification and removal of asymptomatic benign precursor lesions.5 Options available for colorectal cancer screening include FOBT, flexible sigmoidoscopy, double-contrast barium enema, and colonoscopy.
FOBT, which is the most widely used screening modality because of its noninvasiveness and simplicity, is recommended annually for individuals aged 50 years and older.38 Three randomized trials that cumulatively included nearly 50,000 persons aged 70 to 80 years of age demonstrated that annual or biannual screening with FOBT decreases colorectal cancer incidence and death.39 However, FOBT has low sensitivity, limited ability to detect early lesions, and a low rate of compliance with recommended annual testing,38 all of which may be worse among older adults.
The advantage of flexible sigmoidoscopy and colonoscopy is that they provide an opportunity for therapeutic intervention via removal of precancerous disease. Retrospective studies show that sigmoidoscopic screening reduces mortality when performed as recommended every 5 years for adults 50 years and older, but this modality misses half of all proximal cancers. Colonoscopy is considered the gold standard for screening and is recommended every 10 years for asymptomatic adults who are 50 years of age and older.40,41 Although most flexible sigmoidoscopies and colonoscopies are uneventful, older adults may be at greater risk of complications associated with preparation and performance of these procedures, including perforation, electrolyte disturbances, dehydration, bleeding, arrhythmias, and stroke.42-45 These modalities may also be more technically difficult to perform in elderly individuals due to age-related changes in the elasticity and length of the bowel.46 When performing colonoscopy in very elderly persons, the rate of procedural completion is lower and these patients receive only 15% of the expected gain in life expectancy younger patients typically experience with these procedures.46
Rather than discontinuing colon cancer screening at a predefined age, it should be ceased when early detection is unlikely to prolong life. The ACS and the AGS recommend continuing colorectal cancer screening in older adults until they are unlikely to live 5 years or longer or they have a significant comorbidity that precludes treatment.4 These recommendations were substantiated with data from studies of FOBT, which suggest that a difference in colorectal cancer mortality between screened and unscreened persons only becomes evident 5 years after screening. The findings indicate that people with a life expectancy of less than 5 years will not survive long enough to realize the benefits of screening, particularly if they have clinically insignificant disease, yet would still be at risk for potential complications associated with diagnostic and therapeutic procedures.2,10,47
Cervical Cancer
Before the widespread availability of conventional cytology (Pap smears), cervical cancer was the leading cause of cancer death in the United States. It remains the leading cause of cancer death among women in developing countries.48 Risk factors for cervical cancer are black race, not having undergone conventional cytology screening in more than 3 years, early age at first intercourse, multiple sexual partners, smoking history, history of sexually transmitted diseases, and infection with the human papillomavirus (HPV) or human immunodeficiency virus (HIV). For women who have undergone a total hysterectomy or those who have undergone removal of the cervix for benign gynecologic disease, cervical cancer screening is generally not indicated.7,31
Survival from cervical cancer is related to the stage of cancer at diagnosis. Across the age spectrum, most cases of cervical cancer are diagnosed in unscreened or infrequently screened women. Although conventional cytology is the standard screening test, no randomized clinical trials have been conducted to demonstrate that it decreases cervical cancer mortality. Evidence for its use is based on observational studies, which have demonstrated a decreased incidence of cervical cancer since its use became widespread. The USPSTF
recommends that sexually active women with a cervix undergo Pap tests every 1 to 3 years.31
Cervical cancer screening studies typically exclude postmenopausal women, which makes it difficult to quantify the effectiveness of screening in older women.49 Current recommendations for the age at which a woman can cease cervical cancer screening take into consideration the woman’s screening history. The ACS says at age 70 years, postmenopausal women who have not had an abnormal or positive cytology test result within the past 10 years and whose previous three cytology tests were normal can stop screening.7 Women aged 70 years and older in good health who have not been previously screened, have no documentation of prior screening, or are unlikely to have been screened should still be screened.7
Conventional cytology screening for cervical cancer can be more difficult in elderly women with regard to positioning and comfort. Age may also affect the accuracy of results. The squamo-columnar junction tends to migrate further up into the cervical canal as a woman ages, and mucosal atrophy, which may predispose a woman to false-positive cytology results, is common after menopause. False-positives are likely to lead to additional procedures, anxiety, and expense.
Because HPV infection causes most cases of cervical cancer in the United States, HPV testing, which involves a simple blood test, continues to be evaluated as an option for cervical cancer screening. In 2011, investigators working on behalf of the USPSTF conducted a meta-analysis of cervical cancer screening using HPV testing, liquid cytology, or both. The findings led the USPSTF to conclude that evidence was insufficient to recommend for or against routine use of either method as a primary screening modality for cervical cancer and recommended further study.50
To determine the HPV-related risk of cervical disease in postmenopausal women and the value of HPV screening in this population, Ko and associates49 used a database to identify 344 postmenopausal women aged 50 years and older found to have atypical squamous cells of undetermined significance (ASCUS). More than 25% of women in this single-institution, retrospective study tested positive for high-risk HPV, including several who had no history of HPV-related gynecologic disease. Although women younger than 60 years accounted for 56% of ASCUS cases, women aged 65 to 74 years had the highest prevalence of high-risk HPV.49 Ko and associates said physiological changes associated with menopause may affect a woman’s susceptibility and ability to clear an HPV infection. They concluded that conventional cytology and HPV testing to screen for cervical cancer are important for older postmenopausal women and called for additional study regarding the risk of sexually transmitted diseases like HPV in this population.
Prostate Cancer
Prostate cancer is the most commonly diagnosed cancer and the second leading cause of cancer mortality in men within the United States.37 Like many chronic illnesses, its prevalence increases with age. The prevalence of prostate cancer has increased dramatically with the advent of routine screening in men older than 50 years, and odds suggest that one in six men will receive a prostate cancer diagnosis during his lifetime; however, the majority of these individuals will not die from prostate cancer.1 Screening for prostate cancer usually consists of a DRE and a blood test to measure PSA levels. The US Food and Drug Administration approved the PSA test in 1986, and it was widely adopted for prostate cancer screening in the 1990s.
Although the rate of prostate cancer deaths has declined since PSA testing became widespread, it is not clear whether the decline is a direct result of screening or because of better treatment. Reports from two ongoing randomized trials of PSA screening have sought to resolve this question.51-53 The European Randomized Study of Screening for Prostate Cancer included over 150,000 men aged 55 to 74 years who were randomized to receive PSA testing every 4 years or no screening.51,52 The participants were followed for 9 years, and prostate cancer deaths were thought to decrease by 20% in the screening group; however, the number needed to screen was 1410, and 48 would need to be treated to prevent one death. The study did not report any data on the number of adverse effects of prostate cancer treatment. A 13-year follow-up report from the Prostate, Lung, Colorectal, and Ovarian (PLCO) Cancer Screening Trial53 found that annual prostate cancer screening failed to demonstrate a survival benefit. The PLCO trial studied PSA testing in nearly 77,000 men who ranged in age from 55 to 74 years.
Although screening with PSA may be helpful for some men, a major argument against this modality is that it does not distinguish lethal from clinically insignificant disease. Prostate cancer is a heterogeneous disease, and some cancers detected by PSA are low risk and unlikely to become clinically detectable.54 Further evidence of this fact can be found in an autopsy series that reported prostate cancer in two-thirds of men older than 80 years.55 This finding suggests that prostate cancer screening via PSA testing often leads to substantial overdiagnosis of a cancer that may never become clinically significant over a patient’s lifetime, an issue that becomes more relevant as the individual ages and his life expectancy decreases. Overdiagnosis and overtreatment of prostate cancer can incur increased cost, unnecessary treatment, and reduce quality of life due to treatment-related side effects and heightened anxiety.
Screening with PSA testing is ideal because it is a simple, inexpensive, and noninvasive modality; however, the downstream effects of an elevated PSA level can be detrimental to the patient. Elevated PSA levels lead to additional medical visits, often followed by transrectal prostate biopsy, which can cause anxiety, pain, and inconvenience, as well as potential complications such as hematuria and urinary tract infections. When early-stage prostate cancers are diagnosed by such biopsies, treatment options include surgery, radiation, hormone therapy, or watchful waiting, all of which can be associated with worrisome and sometimes dangerous side effects. The most aggressive treatment option for prostate cancer is prostatectomy. Older men are more likely to experience surgical complications from this procedure, with some studies reporting adverse event rates of incontinence and impotence as high as 50%.56 Radiation is the second most common mode of curative therapy and may cause gastrointestinal side effects such as diarrhea and bleeding in addition to incontinence and impotence. Androgen deprivation therapy is also associated with unwanted toxicities, including hot flashes, decreased bone density, and increased risk of heart disease. Such complications may be considered acceptable if the benefits of screening outweigh the harms, but so far the data are less than convincing.
An ideal screening test is one that is both sensitive and specific. PSA levels may rise in response to other conditions common in older men, such as benign prostatic hyperplasia and prostatitis. False-positives in these instances can contribute to stress and anxiety. One study found that men with false-positive PSA screening results reported more problems with sexual function, pain from biopsy, and anxiety about future risk than men who never underwent screening.57 Many attempts have been made to refine how PSA testing is applied in an effort to increase its sensitivity for prostate cancer, including measuring density, velocity, doubling time, and free PSA. The cutoff value for a positive screening result has also been lowered from 4 µg/L to 3 µg/L. Other studies have looked at combining PSA test results with Gleason scores to determine risk of progression. In the Prostate Cancer Prevention Trial,58 biopsies in 15% of men with a normal PSA level were positive for prostate cancer, with quite a few having Gleason scores greater than 7. A Gleason score between 8 and 10 clearly indicates aggressive disease, but it is not possible to determine whether someone with prostate cancer and a Gleason score below 8 will develop significant disease. This underscores the need for a screening tool able to detect preclinical prostate cancer and predict whether it is likely to become life-threatening.
Currently, no major scientific or medical organizations, including the ACS, American Urological Association, American College of Physicians, National Cancer Institute, American Academy of Family Physicians, American College of Preventive Medicine, and USPSTF, support routine testing for prostate cancer. Instead, these groups recommend discussing the potential benefits and limitations of prostate cancer screening with men older than 50 years who have an average risk of prostate cancer and a minimum 10-year life expectancy.37 According to the USPSTF, the risks associated with prostate cancer screening outweigh the benefits in men who are 75 years and older or who have a life expectancy of less than 10 years.59 Despite these recommendations, almost one-third of men with limited life expectancy continue to receive unnecessary PSA tests.60
Patient Preferences
Age affects life expectancy, the behavior of cancers, and the accuracy of some screening tests, which makes extrapolating data for younger adults to older adults complex. Therefore, it becomes increasingly important to consider and include older adults’ healthcare goals and preferences in the decision-making process. Although quality of life is difficult to define, understanding the patient’s perspective on quality of life increases the likelihood that decisions about cancer screening will reflect his or her goals. Forgoing screening for an older individual might be worth considering if sufficient concern exists that screening and further testing or treatment will lead to impaired quality of life.61
Having meaningful discussions about the risks and benefits of screening will maximize informed decision-making. Discussions on cancer screening are important, especially for elderly patients, because inadequate health literacy is associated with lower rates of screening.62 Research suggests most healthy older adults want their physicians to talk with them about these issues.2,61 The following is a list of sample questions to ask your patient:
• Are you the type of person who wants to know about and fight a cancer even if it might never become clinically significant or are you bothered by medical tests/visits to physicians?
• Do you tolerate additional tests well?
• Do you want to live longer at any cost (ie, treatment side effects)?
Despite the uncertainty about the harms and benefits of screening older adults for cancer, rate of compliance with screening guidelines is being used to measure physician performance and does not consider whether contraindications may require a patient or physician to opt out of screening.47 A survey of physicians identified two primary reasons why they often fail to discuss stopping cancer screening with their patients: first, it is too time consuming; second, there are many competing issues during an office visit.63 The literature does not offer much guidance on how physicians should approach ceasing routine cancer screening with their patients. These and other factors may make physicians more likely to screen patients even when it is not indicated and could lead to more harm and unnecessary follow-up.
Conclusion
Although cancer is the second leading cause of death for patients 65 years and older, a survival benefit from cancer screening is not likely to be realized unless the patient’s life expectancy exceeds 5 years. It is therefore best to individualize cancer screening decisions for elderly patients, with careful consideration given to the patient’s life expectancy based not only on age but on comorbid disease, the benefits and harms of the test, the natural course of the screened-for malignancy in older adults, preexisting risk factors, and the patient’s preferences. Cancer screening should be discontinued for patients with severe comorbidities, a limited life expectancy, or poor overall health status.9 Despite the lack of guidance on how to discuss ceasing cancer screening with patients, it may be the best option in some cases and requires careful discussion with patients about evidence-based guidelines and individual preferences. Discontinuing cancer screening for patients who do not stand to benefit allows the clinician to focus remaining office time on preventive health measures that are more likely to benefit the patient, such as screening for falls and various geriatric syndromes.
The authors report no relevant financial relationships.
References
1. American Cancer Society. Cancer facts and figures 2011. Accessed March 9, 2012.
2. Walter LC, Covinsky KE. Cancer screening in elderly patients: a framework for individualized decision making. JAMA. 2001;285(21):2750-2756.
3. He W, Sengupta M, Velkoff VA, DeBarros KA; US Census Bureau. Current Population Reports. 65+ in the United States: 2005. Washington, DC: US Government Printing Office 2005:23-209. www.census.gov/prod/2006pubs/p23-209.pdf. Accessed March 9, 2012.
4. Smith RA, Cokkinides V, Brawley OW. Cancer screening in the United States, 2009: a review of current American Cancer Society guidelines and issues in cancer screening. CA Cancer J Clin. 2009;59(1):27-41.
5. US Preventive Services Task Force. Screening for colorectal cancer: US Preventive Services Task Force recommendation statement. Ann Intern Med. 2008;149(9):627-637.
6. US Preventive Services Task Force. Screening for breast cancer: recommendations and rationale. Ann Intern Med. 2002;137(5 Part 1):344-346.
7. Smith RA, Cokkinides V, Brawley OW. Cancer screening in the United States, 2012: a review of current American Cancer Society Guidelines and current issues in cancer screening. CA Cancer J Clin. 2012. http://onlinelibrary.wiley.com/doi/10.3322/caac.20143/abstract. Accessed March 9, 2012.
8. US Preventive Services Task Force. Screening for prostate cancer: US Preventive Services Task Force recommendation statement. Ann Intern Med. 2008;149(3):185-191.
9. Terret C, Zulian GB, Naiem A, Albrand G. Multidisciplinary approach to the geriatric oncology patient. J Clin Oncol. 2007;25(14):1876-1881.
10. Ko CW, Sonnenberg A. Comparing risks and benefits of colorectal cancer screening in elderly patients. Gastroenterology. 2005;129(4):1163-1170.
11. Yancik R. Cancer burden in the aged: an epidemiologic and demographic overview. Cancer. 1997;80(7):1273-1283.
12. Satariano WA, Ragland DR. The effect of comorbidity on 3-year survival of women with primary breast cancer. Ann Intern Med. 1994;120(2):104-110.
13. Satariano WA, Silliman RA. Comorbidity: implications for research and practice in geriatric oncology.Crit Rev Oncol Hematol. 2003;48(2):239-248.
14. Gross CP, McAvay GJ, Krumholz HM, Paltiel AD, Bhasin D, Tinetti ME. The effect of age and chronic illness on life expectancy after a diagnosis of colorectal cancer: implications for screening. Ann Intern Med. 2006;145(9):646-653.
15. Linn BS, Linn MW, Gurel L. Cumulative illness rating scale. J Am Geriatr Soc. 1968;16(5):622-626.
16. American Geriatrics Society. Breast cancer screening in older women (reviewed and updated in 2005). Accessed March 9, 2012.
17. Ziogas A, Horick NK, Kinney AY, et al. Clinically relevant changes in family history of cancer over time. JAMA. 2011;306(2):172-178. http://jama.ama-assn.org/content/306/2/172.full.pdf. Accessed March 9, 2012.
18. Lee SJ, Go AS, Lindquist K, Bertenthal D, Covinsky KE. Chronic conditions and mortality among the oldest old. Am J Public Health. 2008;98(7):1209-1214.
19. Extermann M, Overcash J, Lyman GH, Parr J, Balducci L. Comorbidity and functional status are independent in older cancer patients. J Clin Oncol. 1998;16(4):1582-1587.
20. US Census Bureau. Population profile of the United States. The elderly population. Accessed March 9, 2012.
21. Balducci L, Ershler WB, Lyman GH, Extermann M, eds. Comprehensive Geriatric Oncology. 2nd ed. Boca Raton, FL: Taylor & Francis, 2004:225.
22. Mushlin AI, Kouides RW, Shapiro DE. Estimating the accuracy of screening mammography: a meta-analysis. Am J Prev Med. 1998;14(2):143-153.
23. Walter LC, Eng C, Covinsky KE. Screening mammography for frail older women: what are the burdens? J Gen Intern Med. 2001;16(11):779-784.
24. Winawer SJ, Fletcher RH, Miller L, et al. Colorectal cancer screening: clinical guidelines and rationale. Gastroenterology. 1997;112(2):594-642.
25. Day LW, Kwon A, Inadomi JM, Walter LC, Somsouk M. Adverse events in older patients undergoing colonoscopy: a systematic review and meta-analysis. Gastrointest Endosc. 2011;74(4):885-896.
26. Berry DA, Cronin KA, Plevritis SK, et al; Cancer Intervention and Surveillance Modeling Network Collaborators. Effect of screening and adjuvant therapy on mortality from breast cancer. N Engl J Med. 2005;353(17):1784-1792.
27. Walter LC, Lewis CL, Barton MB. Screening for colorectal, breast, and cervical cancer in the elderly: a review of the evidence. Am J Med. 2005;118(10):1078-1086.
28. Fletcher SW, Black W, Harris R, Rimer BK, Shapiro S. Report of the International Workshop on Screening for Breast Cancer. J Natl Cancer Inst. 1993;85(20):1644-1656.
29. Elwood JM, Cox B, Richardson AK. The effectiveness of breast cancer screening by mammography in younger women. Online J Curr Clin Trials. 1994. www.ncbi.nlm.nih.gov/pubmed/8305999. Accessed March 9, 2012.
30. Olsen O, Gøtzsche PC. Screening for breast cancer with mammography [update appears in Cochrane Database Syst Rev. 2006;(4):CD001877]. Cochrane Database Syst Rev. 2001;(4):CD001877.
31. US Preventive Services Task Force. Recommendations for adults: cancer. Rockville, MD: US Preventive Services Task Force; 2011. Accessed February 27, 2012.
32. Smith-Bindman R, Kerlikowske K, Gebretsadik T, Newman J. Is screening mammography effective in elderly women? Am J Med. 2000;108(2):112-119.
33. Clark GM. The biology of breast cancer in older women. J Gerontol. 1992;47:19-23.
34. Mathieu E, Barratt A, Davey HM, McGeechan K, Howard K, Houssami N. Informed choice in mammography screening: a randomized trial of a decision aid for 70-year-old women. Arch Intern Med. 2007;167(19):2039-2046.
35. Fletcher SW, Elmore JG. Clinical practice. Mammographic screening for breast cancer. N Engl J Med. 2003;348(17):1672-1680.
36. Silverman MA, Zaidi U, Barnett S, et al. Cancer screening in the elderly population. Hematol Oncol Clin North Am. 2000;14(1):89-112, ix.
37. American Cancer Society. Cancer facts and figures 2008. www.cancer.org/
Research/CancerFactsFigures/CancerFactsFigures/2008cafffinalsecured-pdf. Accessed February 23, 2012.
38. US Centers for Disease Control and Prevention. Colorectal cancer test use among persons aged ≥50 years—United States, 2001. MMWR Morb Mortal Wkly Rep. 2008;57(10):253-258.
39. Pignone M, Rich M, Teutsch SM, Berg AO, Lohr KN. Screening for colorectal cancer in adults at average risk: a summary of the evidence for the US Preventive Services Task Force. Ann Intern Med. 2002;137(2):132-141.
40. Levin B, Lieberman DA, McFarland B, et al. Screening and surveillance for the early detection of colorectal cancer and adenomatous polyps, 2008: a joint guideline from the American Cancer Society, the US Multi-Society Task Force on Colorectal Cancer, and the American College of Radiology. Gastroenterology. 2008;134(5):1570-1595.
41. Winawer SJ, Flehinger BJ, Schottenfeld D, Miller DG. Screening for colorectal cancer with fecal occult blood testing and sigmoidoscopy. J Natl Cancer Inst. 1993;85(16):1311-1318.
42. Arora A, Singh P. Colonoscopy in patients 80 years of age and older is safe, with high success rate and diagnostic yield. Gastrointest Endosc. 2004;60(3):408-413.
43. Chatrenet P, Friocourt P, Ramain JP, Cherrier M, Maillard JB. Colonoscopy in the elderly: a study of 200 cases. Eur J Med. 1993;2(7):411-413.
44. Lukens FJ, Loeb DS, Machicao VI, Achem SR, Picco MF. Colonoscopy in octogenarians: a prospective outpatient study. Am J Gastroenterol. 2002;97(7):1722-1725.
45. Ure T, Dehghan K, Vernava AM 3rd, Longo WE, Andrus CA, Daniel GL. Colonoscopy in the elderly. Low risk, high yield. Surg Endosc. 1995;9(5):505-508.
46. Lin OS, Kozarek RA, Schembre DB, et al. Screening colonoscopy in very elderly patients: prevalence of neoplasia and estimated impact on life expectancy. JAMA. 2006;295(20):2357-2365.
47. Walter LC, Lindquist K, Nugent S, et al. Impact of age and comorbidity on colorectal cancer screening among older veterans. Ann Intern Med. 2009;150(7):465-473.
48. Alliance for Cervical Cancer Prevention. Preventing cervical cancer worldwide. 2004. http://bit.ly/ACCP-Preventing-CC. Accessed March 9, 2012.
49. Ko EM, Tambouret R, Wilbur D, Goodman A. HPV reflex testing in menopausal women. Patholog Res Int. 2011:181870. www.ncbi.nlm.nih.gov/pmc/articles/PMC3090035/?tool=pubmed. Accessed March 9, 2012.
50. Whitlock EP, Vesco KK, Eder M, Lin JS, Senger CA, Burda BU. Liquid-based cytology and human papillomavirus testing to screen for cervical cancer: a systematic review for the US Preventive Services Task Force. Ann Intern Med. 2011;155(10):687-697.
51. Schröder FH, Hugosson J, Roobol MJ, et al; ERSPC Investigators. Screening and prostate-cancer mortality in a randomized European study. N Engl J Med. 2009;360(13):1320-1328.
52. Barry MJ. Screening for prostate cancer—the controversy that refuses to die. N Engl J Med. 2009;360(13):1351-1354.
53. Andriole GL, Crawford ED, Grubb RL 3rd, et al; for the PLCO Project Team. Prostate cancer screening in the Randomized Prostate, Lung, Colorectal, and Ovarian Cancer Screening Trial: mortality results after 13 years of follow-up. J Natl Cancer Inst. 2012;104(2):125-132.
54. Draisma G, Etzioni R, Tsodikov A, et al. Lead time and overdiagnosis in prostate-specific antigen screening: importance of methods and context. J Natl Cancer Inst. 2009;101(6):374-383.
55. Breslow N, Chan CW, Dhom G, et al. Latent carcinoma of prostate at autopsy in seven areas. The International Agency for Research on Cancer, Lyons, France. Int J Cancer. 1977;20(5):680-688.
56. Harris R, Lohr KN. Screening for prostate cancer: an update of the evidence for the US Preventive Services Task Force. Ann Intern Med. 2002;137(11):917-929.
57. Katz DA, Jarrard DF, McHorney CA, Hillis SL, Wiebe DA, Fryback DG. Health perceptions in patients who undergo screening and workup for prostate cancer. Urology. 2007;69(2):215-220.
58. Thompson IM, Pauler DK, Goodman PJ, et al. Prevalence of prostate cancer among men with a prostate-specific antigen level ≤4.0 ng per milliliter [published correction appears in N Engl J Med. 2004;351(14):1470]. N Engl J Med. 2004;350(22):2239-2246.
59. Fowler FJ Jr, Barry MJ, Walker-Corkery B, et al. The impact of a suspicious prostate biopsy on patients’ psychological, socio-behavioral, and medical care outcomes. J Gen Intern Med. 2006;21(7):715-721.
60. Drazer MW, Huo D, Schonberg MA, Razmaria A, Eggener SE. Population-based patterns and predictors of prostate-specific antigen screening among older men in the United States. J Clin Oncol. 2011;29(13):1736-1743.
61. Kaplan MH, Feinstein AR. The importance of classifying initial comorbidity in evaluating the outcome of diabetes mellitus. J Chronic Dis. 1974;27(7-8):387-404.
62. Baker DW, Wolf MS, Feinglass J, Thompson JA, Gazmararian JA, Huang J. Health literacy and mortality among elderly persons. Arch Intern Med. 2007;167(14):1503-1509.
63.Schonberg MA, Ramanan RA, McCarthy EP, Marcantonio ER. Decision making and counseling around mammography screening for women aged 80 or older. J Gen Intern Med. 2006;21(9):979-985.


